The validation lifecycle is a critical concept in Computer System Validation (CSV) and Quality Systems. Whether you’re in pharmaceuticals, biotech, medical devices, or regulated digital health, understanding the lifecycle from User Requirements Specification (URS) to Performance Qualification (PQ) is essential for compliance, audit readiness, and product integrity.
The goal is simple: prove that a computerized system is fit for its intended use — consistently, reliably, and in compliance with regulatory expectations
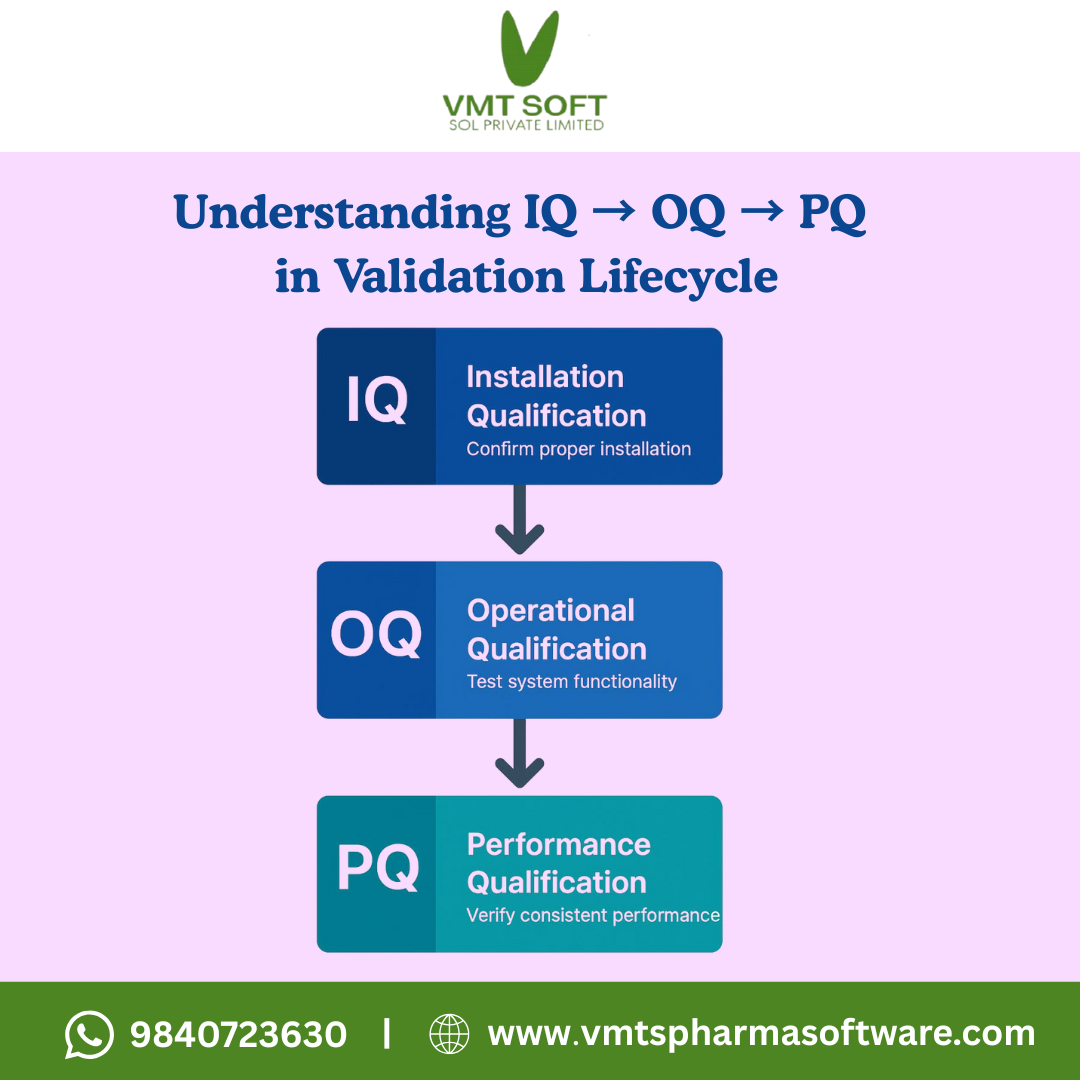
Here’s the simple visual lifecycle flow used in most CSV and GxP environments:
➡️ URS → FS/DS → IQ → OQ → PQ → Release
Let’s break it down.
User Requirements Specification (URS)
The URS defines what the system must do, from business needs to compliance expectations.
Common inclusions:
URS forms the foundation for the rest of the validation lifecycle.
Installation Qualification (IQ)
IQ verifies that the system is correctly installed and documented.
Includes validation of:
Performance Qualification (PQ)
PQ tests whether the system performs correctly in real-world conditions.
It validates:
If the system passes PQ, it is considered fit for intended use — meeting GxP compliance expectations.
A proper validation lifecycle reduces compliance risks and ensures:
In regulated environments, validation isn’t optional — it’s a required control under GxP and quality systems.
Functional Specification / Design Specification (FS/DS)
Once the URS is approved, it is translated into:
Document | Purpose |
FS (Functional Specification) | Defines what functions the system must include. |
DS (Design Specification) | Defines how those functions will be implemented. |
These documents support traceability and are essential for later testing.
Operational Qualification (OQ)
OQ confirms that the system operates correctly under expected conditions.
Testing focuses on:
Once all stages are complete, the system is:
But validation does not end here.
Ongoing activities include:
This ensures continuous compliance throughout the system lifecycle.
With AI-enabled QMS platforms, cloud environments, and increasing regulatory expectations, the validation lifecycle ensures:
It is one of the most important elements of modern CSV lifecycle management and quality systems.
The validation lifecycle (URS → PQ → Release) is more than a documentation exercise — it is a structured process that ensures systems are:
Organizations that follow this lifecycle are better positioned for audits, regulatory inspections, and digital transformation success.
If your organization needs support with:
📩 Connect with us — we help teams stay inspection-ready.
VMT Soft Sol Pvt. Ltd. specializes in eQMS Software for the pharmaceutical industry. We simplify compliance, enhance efficiency, and ensure operational excellence.
# 8-3-167/A/52, 2nd Floor, Vani Nilayam, Vikaspuri, AG Colony Road, S R Nagar(PO), Hyderabad-500038.
Phone Number : +91-9866968830
